Cancer could be detected 3 years before symptoms appear with a simple blood test; new study reveals
A groundbreaking study by Johns Hopkins University researchers indicates that a simple blood test could detect cancer up to three years before any symptoms manifest. This innovation may revolutionize early diagnosis and prevention strategies for this deadly disease.
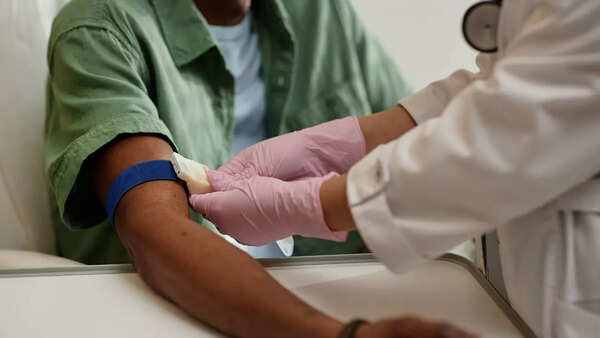
Published in the journal Cancer Discovery, the study highlights the potential of early cancer detection to dramatically improve survival rates and expand treatment options. Currently, late diagnosis remains a critical challenge in cancer treatment, contributing to millions of deaths annually.
The Potential of Early Detection
Early detection is paramount in effectively combating cancer. When tumors are identified in their initial stages, they are typically smaller, less aggressive, and more responsive to treatment. Researcher Yuxuan Wang emphasizes that a three-year lead time allows for timely intervention, potentially transforming a life-threatening condition into a curable one.
This advantage could be particularly crucial in managing aggressive forms of cancer, making the difference between a successful recovery and a grim prognosis.
Unlocking the Science Behind Blood Detection
The study focuses on circulating tumor DNA (ctDNA), which tumors naturally release into the bloodstream. These DNA fragments are often present in extremely low concentrations, making them difficult to detect, especially in the early stages of cancer.

To overcome this challenge, the researchers employed sophisticated algorithms to analyze blood samples for specific DNA modifications associated with tumors. This technique forms the basis of a Multi-Cancer Early Detection (MCED) test, designed to identify cancer-specific genetic alterations in the blood.
The research team analyzed blood samples from 52 participants, divided into two groups:
- 26 individuals who were diagnosed with cancer within six months of sample collection.
- 26 individuals who remained cancer-free.
The MCED test successfully flagged eight of the cancer cases, achieving a 31% detection rate. Importantly, this detection occurred before any formal diagnosis or the appearance of noticeable symptoms.
Study Findings: A Glimpse into the Future
Further analysis of older blood samples from some participants revealed even more promising results. Among the eight individuals detected by the MCED test, six had blood samples available from 3.1 to 3.5 years prior to their diagnosis. Cancer signals were identified in four of these six samples, indicating the presence of ctDNA, albeit at levels significantly lower than the current test's detection threshold.

These findings suggest that tumors begin shedding DNA into the bloodstream long before symptoms become apparent, and that with enhanced test sensitivity, these early signs could be detected. While the results are encouraging, they also underscore the need to improve the sensitivity of current technology to detect lower levels of ctDNA in early-stage cancer.
Dr. Bert Vogelstein, a senior cancer researcher involved in the project, notes, "This study shows the promise of MCED tests in detecting cancers very early. But it also sets the benchmark sensitivities required for these tests to succeed."
Navigating the Path Forward
Despite the scientific advancements, transitioning this technology from the lab to clinical practice requires further rigorous clinical trials to ensure reliability and safety. Regulatory approvals are also necessary before these tests can be widely adopted.
Moreover, protocols must be established for managing positive test results, including determining the appropriate clinical follow-up procedures such as scans, biopsies, and preventive treatments.
Dr. Nickolas Papadopoulos from the Ludwig Centre emphasizes the importance of determining the appropriate clinical follow-up after a positive test result.
Despite these challenges, this research signifies a significant and hopeful step forward in cancer diagnostics. Combined with ongoing advancements in treatment, particularly therapies targeting multiple cancer types, the future holds the potential for significantly improved survival rates. This innovative approach could revolutionize how cancer is screened and treated, offering new hope in the fight against this devastating disease.
Newer articles
Older articles
-
 Mahbub Anam replaces Faruque Ahmed as new BPL chairman
Mahbub Anam replaces Faruque Ahmed as new BPL chairman
-
 Microsoft plans to take on iPhone and Android smartphones with this new device
Microsoft plans to take on iPhone and Android smartphones with this new device
-
 This new AI tool can help you book train tickets, get refunds and check details on IRCTC website and app
This new AI tool can help you book train tickets, get refunds and check details on IRCTC website and app
-
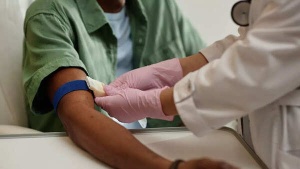 Cancer could be detected 3 years before symptoms appear with a simple blood test; new study reveals
Cancer could be detected 3 years before symptoms appear with a simple blood test; new study reveals
-
 Ngarava, Curran and Raza to miss Test series against South Africa
Ngarava, Curran and Raza to miss Test series against South Africa
-
 IND vs ENG: How to bat in England? Sachin Tendulkar shares tips for inexperienced Indian batting
IND vs ENG: How to bat in England? Sachin Tendulkar shares tips for inexperienced Indian batting
-
 NASA astronauts prepare ‘space sushi’ aboard the ISS in zero gravity during a heartwarming crew celebration
NASA astronauts prepare ‘space sushi’ aboard the ISS in zero gravity during a heartwarming crew celebration
-
 Don't worry about the outside world: Tendulkar's advice to Gill
Don't worry about the outside world: Tendulkar's advice to Gill
-
 Mehidy set to return for second Test in Colombo
Mehidy set to return for second Test in Colombo
-
 Twitter bans over 5 lakh accounts in India, here's why
Twitter bans over 5 lakh accounts in India, here's why